
Whereas scientists have lengthy identified that iron oxide minerals assist lock away monumental quantities of carbon—sequestering it from the ambiance—a brand new Northwestern College examine now reveals precisely why these minerals are such highly effective carbon traps.
By exploring ferrihydrite, a standard iron oxide mineral, engineers found it employs a number of, essentially totally different chemical methods to seize carbon and lock it away.
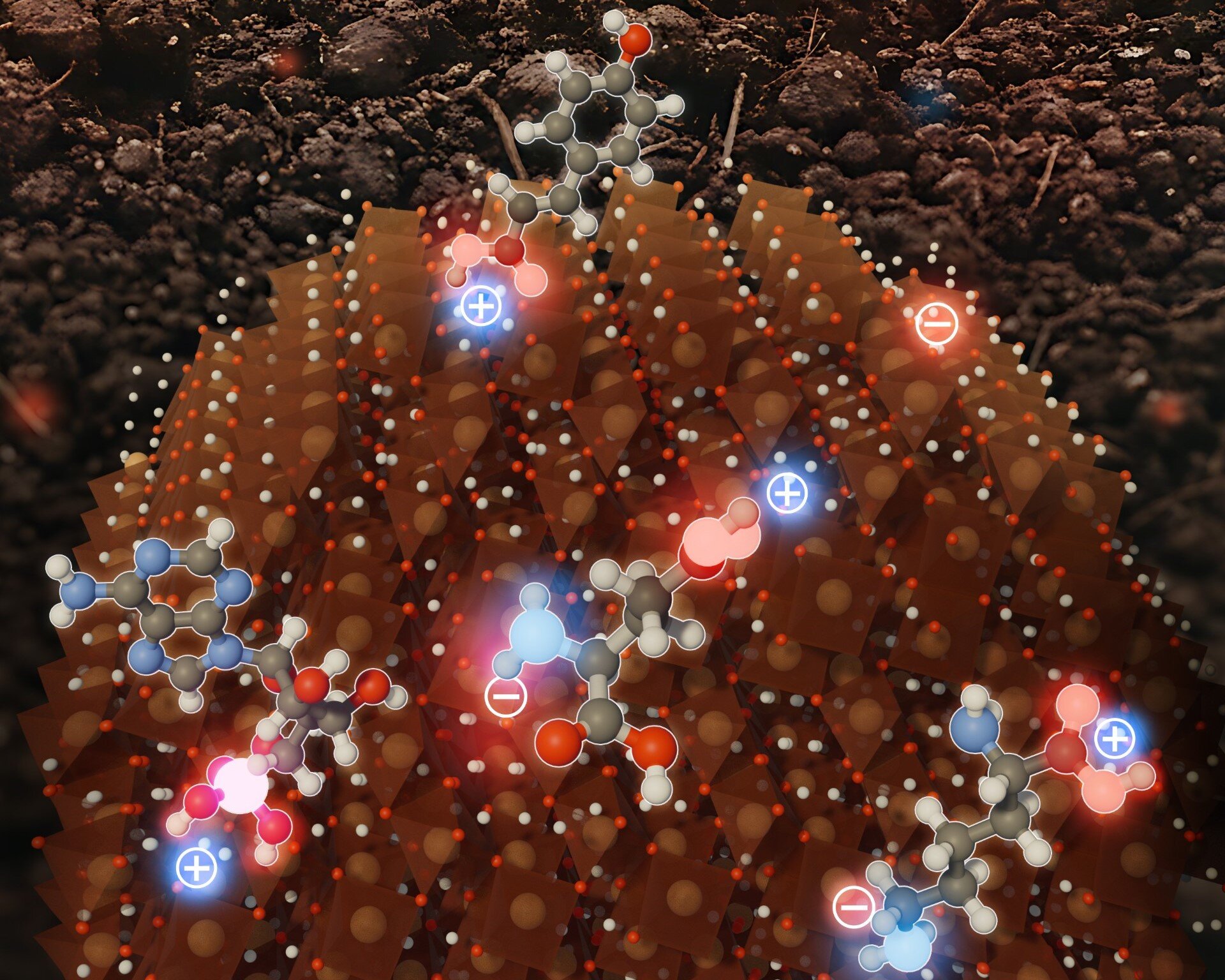
Though ferrihydrite has an general constructive electrical cost, the engineers discovered its floor is just not uniformly charged. As an alternative, its floor resembles a nanoscale mosaic of positively and negatively charged patches. And ferrihydrite doesn’t entice carbon utilizing electrostatic attraction alone. It additionally makes use of chemical bonds and hydrogen bonding to type sturdy chemical hyperlinks between its floor and natural supplies.
These surprising methods flip iron oxide minerals into extremely versatile carbon snatchers, able to grabbing and holding onto many various kinds of natural molecules. The findings provide new perception into how these minerals in soils entice carbon for many years and even centuries, stopping it from coming into the ambiance as climate-warming greenhouse gases.
The examine was printed within the journal Environmental Science & Expertise. The analysis gives probably the most detailed look but on the floor chemistry of ferrihydrite, an necessary sort of iron oxide minerals.
“Iron oxide minerals are necessary for controlling the long-term preservation of natural carbon in soils and marine sediments,” stated Northwestern’s Ludmilla Aristilde, who led the examine. “The destiny of natural carbon within the setting is tightly linked to the worldwide carbon cycle, together with the transformation of natural matter to greenhouse gases. Subsequently, it is necessary to grasp how minerals entice natural matter, however the quantitative analysis of how iron oxides entice various kinds of natural matter by way of totally different binding mechanisms has been lacking.”
An professional within the dynamics of organics in environmental processes, Aristilde is a professor of civil and environmental engineering at Northwestern’s McCormick College of Engineering. She can be a member of the Worldwide Institute for Nanotechnology, the Paula M. Trienens Institute for Sustainability and Power and Middle for Artificial Biology. Jiaxing Wang is the examine’s first creator, and Benjamin Barrios Cerda is the examine’s second creator. Each Wang and Barrios Cerda are at the moment postdoctoral associates in Aristilde’s laboratory.
Maintaining carbon buried
Holding roughly 2,500 billion tons of sequestered carbon, soil is certainly one of Earth’s largest carbon sinks—second solely to the ocean. However regardless that soil is throughout us, scientists are solely simply starting to grasp the way it locks in carbon to take away it from the lively carbon cycle.
By combining laboratory experiments with theoretical modeling, Aristilde and her group have spent years finding out minerals and soil-dwelling microbes with the purpose of figuring out the elements that trigger soil to both entice or launch carbon. In earlier works, Aristilde and her group explored how clay minerals bind natural matter and the way soil microbes preferentially flip non-sugar organics into carbon dioxide.
Within the new examine, Aristilde’s group turned its focus to iron oxide minerals, that are related to greater than one-third of the natural carbon saved in soils. Particularly, the group examined ferrihydrite, a sort of iron oxide mineral generally present in soils close to plant roots or in soils and sediments with plentiful natural matter. Though ferrihydrite seems to be positively charged below many environmental circumstances, it manages to bind all kinds of natural compounds—some negatively charged, some positively charged and a few impartial.
Watching molecules stick
To grasp how this happens, Aristilde and her group first used high-resolution molecular modeling and atomic pressure microscopy to realize an in depth take a look at the mineral’s floor. Whereas the mineral’s cost is constructive general, the researchers discovered its floor truly comprises intermixed patches of constructive and detrimental expenses. The discovering explains why ferrihydrite can appeal to negatively charged species like phosphate and positively charged species like metallic ions.
“It’s effectively documented that the general cost of ferrihydrite is constructive in related environmental circumstances,” Aristilde stated. “That has led to assumptions that solely negatively charged compounds will bind to those minerals, however we all know the minerals can bind compounds with each detrimental and constructive expenses. Our work illustrates that it’s the sum of each detrimental and constructive expenses distributed throughout the floor that provides the mineral its general constructive cost.”
After mapping ferrihydrite’s floor expenses, Aristilde and her group examined how molecules bind to it, permitting them to attach floor chemistry on to carbon trapping. They launched ferrihydrite to natural molecules generally present in soils, together with amino acids, plant acids, sugars and ribonucleotides. Then, they measured how a lot of those molecules caught to the ferrihydrite and used infrared spectroscopy to look at precisely how every molecule connected.
Greater than attraction
In the end, the group discovered that compounds bind to ferrihydrite utilizing a number of methods. Whereas positively charged amino acids bonded to detrimental patches on ferrihydrite’s floor, negatively charged amino acids bonded to the positively charged patches. Different compounds, like ribonucleotides, are first drawn to ferrihydrite by electrostatic attraction after which go on to type a lot stronger chemical bonds with iron atoms. And sugars, which type the weakest bonds, are connected to the mineral by way of hydrogen bonding.
“Collectively, our findings present a rationale, on a quantitative foundation, for constructing a framework for the mechanisms that drive mineral-organic associations involving iron oxides within the long-term preservation of natural matter,” Aristilde stated. “These associations could assist clarify why some natural molecules stay protected in soils whereas others are extra susceptible to being damaged down and respired by microbes.”
Subsequent, the group plans to analyze what occurs after natural molecules are connected to mineral surfaces. Some compounds could endure chemical transformations to merchandise which can be out there for additional degradation or to much more steady merchandise that may very well be proof against decomposition.
Extra data:
Jiaxing Wang et al, Floor Cost Heterogeneity and Mechanisms of Natural Binding Modes on an Iron Oxyhydroxide, Environmental Science & Expertise (2025). DOI: 10.1021/acs.est.5c10850
Supplied by
Northwestern College
Quotation:
Iron minerals’ hidden chemistry explains how soils entice carbon (2025, December 15)
retrieved 16 December 2025
from https://phys.org/information/2025-12-iron-minerals-hidden-chemistry-soils.html
This doc is topic to copyright. Aside from any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.
Leave a Reply